Gene Therapy for Hemophilia: A New Era of Healing
Gene therapy for hemophilia is revolutionizing the way this genetic bleeding disorder is managed, offering hope to thousands affected by the condition. Hemophilia B, a rare illness stemming from a deficiency in clotting factor IX, has traditionally required patients to undergo regular injections of clotting factors to mitigate bleeding risks. However, recent advancements, exemplified by the FDA gene therapy approval of Hemgenix, have introduced a groundbreaking treatment option with the potential for long-term relief from daily management. This innovative approach not only aims to reduce the frequency of infusion but also represents a significant leap towards gene therapy success in hemophilia treatment. As we delve into this transformative journey, it becomes clear that gene therapy for hemophilia could reshape the landscape of hemophilia management for current and future patients alike.
The emergence of genetic treatments for hemophilia has paved the way for a new era in disease management, marking a pivotal shift in how we approach this lifelong condition. Hemophilia, particularly hemophilia B, involves a lack of factor IX, leading to challenges in blood clotting and significant life impacts for those affected. The advent of innovative therapies like Hemgenix has opened up conversations about curative possibilities and the potential to elevate living standards for patients. As more individuals explore these advanced treatment options, the field of hemophilia care is witnessing a significant transformation that aligns with evolving medical technologies and patient needs. By leveraging the power of gene therapy, patients may finally gain a chance to break free from the constraints of traditional clotting factor therapies.
Overview of Gene Therapy for Hemophilia
Gene therapy for hemophilia represents a groundbreaking advance in the management of this genetic disorder, particularly hemophilia B. By introducing functional copies of the gene responsible for producing clotting factor IX, therapies like Hemgenix aim to reduce or eliminate the need for regular factor infusions. This innovative approach not only has the potential to enhance the quality of life for patients but also seeks to provide a long-term solution instead of merely managing the symptoms associated with hemophilia.
Recent advancements in gene therapy have been fueled by significant research and positive clinical trial results. The approval of Hemgenix by the FDA in 2022 is a testament to the promising future of gene therapy options. Patients are cautiously optimistic as they anticipate a change from daily injections to a one-time treatment that could significantly reduce their risk of spontaneous bleeding, a common event in people with hemophilia.
The Impact of FDA Gene Therapy Approval on Treatment Options
The FDA’s approval of gene therapies like Hemgenix is pivotal in expanding treatment options for patients with hemophilia. This approval not only signifies regulatory recognition but also encourages further research into gene therapy modalities. With a clearer path for market entry, biopharmaceutical companies are more motivated to invest in research and development, enhancing the innovation landscape for hemophilia treatments.
Additionally, FDA approval can help in alleviating some financial burdens typically associated with chronic hemophilia management. As insurance companies begin to recognize the long-term benefits of a one-time gene therapy treatment over ongoing costly factor replacement therapy, patients may find it easier to access these novel treatments. This progress promises to alleviate the continuous worry often tied to managing this condition, offering patients like Terence Blue a chance at a more liberated lifestyle.
Exploring Hemophilia B Treatment Options
Hemophilia B treatment has significantly evolved over the past few decades, moving from regular infusions of clotting factor to innovative therapies like gene therapy. Traditional treatments focused on prophylactic infusions of factor IX, which were essential in preventing bleeding episodes. However, these treatments often require a strict adherence schedule and can lead to complications associated with repeated infusions.
With gene therapy, patients are provided with an opportunity to address the underlying genetic defect directly. Hemgenix aims to prompt the body to produce its own clotting factor, substantially decreasing reliance on regular treatments. This shift represents a monumental change in how hemophilia can be managed, addressing both the physical and emotional challenges that accompany a lifetime of treatment.
Understanding the Mechanism Behind Gene Therapy
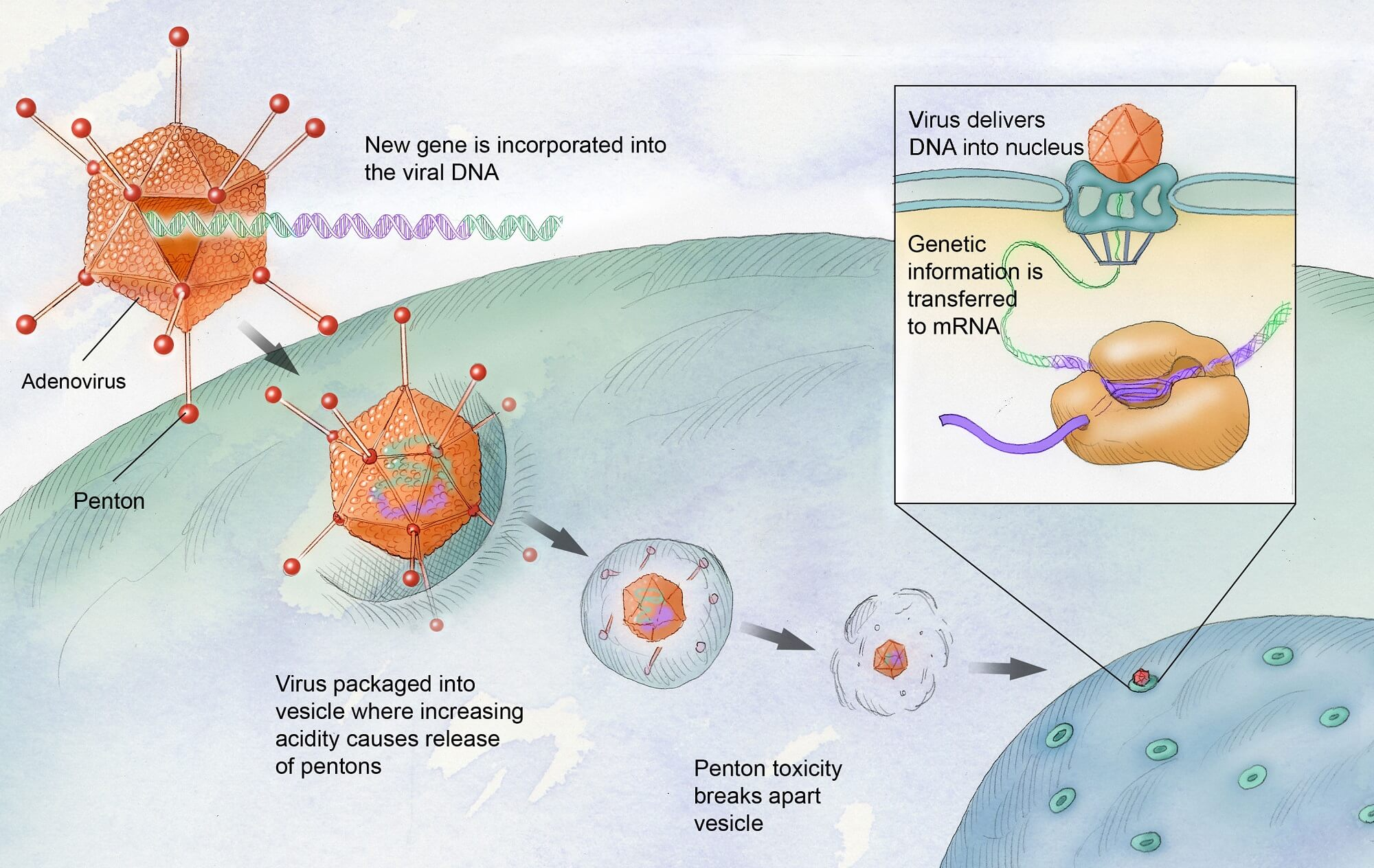
Gene therapy works by leveraging viruses to deliver healthy genes into the patient’s cells. In the case of hemophilia B, specifically designed vectors target liver cells, where clotting factors are produced. By correcting the genetic mutation responsible for clotting factor IX deficiency, patients can potentially increase their factor IX production, thereby reducing their risk for spontaneous bleeds.
This approach utilizes a ‘Trojan horse’ mechanism, allowing patients to receive treatment through a relatively simple outpatient procedure. This innovative mechanism is not only effective but also encourages patient acceptance of the treatment as it circumvents the need for frequent injections — a common barrier to long-term treatment compliance.
Long-Term Effects of Gene Therapy on Patient Health
The long-term efficacy of gene therapy for hemophilia, such as Hemgenix, offers hope for improved patient outcomes. Clinical trials have demonstrated that a significant percentage of recipients do not need factor IX prophylaxis years after treatment. In practical terms, this means many may avoid the burden of managing a chronic condition, leading to a better quality of life and reduced healthcare costs.
Patients express excitement at the prospect of living with fewer restrictions. As they experience fewer bleeds, they can engage in physical activities they previously avoided due to their hemophilia. This transformative potential of gene therapy is reshaping the landscape of hemophilia management, moving towards an era where managing the condition could become more straightforward and less anxiety-provoking.
Challenges in the Implementation of Gene Therapy
Despite the promise of gene therapy for hemophilia, several challenges remain in the implementation of these treatments. High costs associated with development and treatment can limit patient access. Even as insurance negotiations take place, the price point of therapies like Hemgenix remains daunting for many, necessitating systemic changes in how new gene therapies are priced and made available.
Additionally, patient education plays a crucial role in the acceptance of gene therapy. Many patients express hesitations regarding new treatments, needing thorough explanations and shared experiences before committing to a therapy that involves genetically altering their own cells. Overcoming these educational barriers will be essential to foster a greater acceptance of gene therapies as viable and necessary options.
The Emotional and Social Impact of Hemophilia Management
The emotional toll of living with hemophilia is substantial, as patients often grapple with feelings of isolation and inadequacy due to their condition. The daily regimen of infusions can serve as a constant reminder of their limitations, leading to anxiety and depression. Treatments that provide a sense of normalcy and freedom, such as gene therapy, can substantially improve mental health outcomes for patients.
For individuals like Terence Blue, the thought of potentially freeing themselves from the daily needle routine is not merely a medical decision; it is a means of reclaiming their life. Social interactions improve as patients feel less constrained by their health, thus positively impacting relationships and overall well-being. Consequently, gene therapy’s potential to alleviate both the physical and emotional burdens of hemophilia management is a critical aspect of its importance.
The Future of Gene Therapy in Hemophilia Management
As research and technology advance, the future of gene therapy for hemophilia looks promising. Scientists are continually refining methods to enhance gene delivery and improve the longevity of treatment effects. Newer therapies are expected to emerge, potentially targeting other forms of hemophilia and similar bleeding disorders, thereby broadening the spectrum of diseases that can be treated with gene therapy.
Furthermore, the prospect of gene therapy could lead to significant improvements in patient outcomes and healthcare economics. With successful outcomes from gene therapies, better pricing models and broader patient access are likely to result, making groundbreaking treatments available to a larger population. This anticipation is driving the ongoing research and development efforts aimed at unlocking the full potential of gene therapy in hemophilia management.
Patient Case Studies: Success Stories with Gene Therapy
Real-life success stories, such as that of Terence Blue, are beginning to emerge as gene therapy becomes more prevalent in hemophilia treatment. Clinical experiences highlight how patients experience increased factor production and fewer complications following treatment, showcasing the therapy’s ability to transform lives. Terence’s progress, including a significant rise in his factor IX levels, illustrates the success of gene therapy and instills hope among others living with similar health challenges.
As more patients undergo gene therapy and share their experiences, a growing body of evidence supports the effectiveness and safety of these treatments. These case studies play an essential role in building the trust needed for widespread adoption. As seen with Terence, the emotional uplift and freedom from medical ties often encourage others to pursue similar treatments, potentially changing the landscape of hemophilia management for future generations.
Frequently Asked Questions
What is gene therapy for hemophilia and how does it work?
Gene therapy for hemophilia aims to introduce a corrected copy of the gene responsible for producing clotting factor, specifically for conditions like hemophilia B. The therapy uses a modified virus to deliver this healthy gene into the liver cells, which then start producing the clotting factor IX that is deficient in patients.
Can gene therapy be considered a cure for hemophilia B treatment?
While gene therapy for hemophilia B, such as Hemgenix, shows promising results and can significantly reduce or eliminate the need for regular clotting factor infusions, it is not yet universally classified as a ‘cure.’ However, many patients experience long-term benefits, with a high percentage not requiring ongoing treatment.
What are the benefits of gene therapy in hemophilia management?
Gene therapy for hemophilia management offers numerous benefits, including reduced frequency of bleeding episodes, decreased need for regular injections of clotting factor, and improved overall quality of life. Patients also report less anxiety and more freedom from daily treatment routines.
How has FDA approval impacted gene therapy for hemophilia?
The FDA approval of gene therapy for hemophilia, such as Hemgenix, has opened doors for new treatment options, enabling more patients access to innovative therapies. This approval signifies a critical step in bringing effective gene therapies to patients and encourages further research and development in the field.
What is the cost of gene therapy for hemophilia, and how is it managed?
Gene therapy for hemophilia can be quite expensive, with treatments like Hemgenix costing around $3.5 million. However, insurance companies typically negotiate prices, and financial assistance programs may be available to help manage these costs.
Are there any risks associated with gene therapy for hemophilia?
While gene therapy for hemophilia generally has a good safety profile, potential risks include immune responses to the therapeutic virus or liver complications. Patients undergo thorough screening and monitoring to mitigate these risks during treatment.
What advancements have been made in gene therapy for hemophilia over the years?
Significant advancements in gene therapy for hemophilia have been made in recent years, including the development of safer viral vectors for gene delivery, improved understanding of gene modifications, and successful clinical trials demonstrating effectiveness and long-term benefits.
How does gene therapy for hemophilia compare to traditional treatment methods?
Compared to traditional treatment methods, which involve regular infusions of clotting factor, gene therapy for hemophilia offers a potentially one-time solution with long-lasting results, thereby reducing the burden of frequent treatments and improving patients’ lifestyles.
What should patients consider before opting for gene therapy for hemophilia?
Patients should consider discussing their specific health conditions, potential benefits, risks, and the nature of gene therapy with their healthcare providers. It’s also essential to review the availability of support, insurance coverage, and long-term follow-up requirements.
What is the success rate of gene therapy for hemophilia in clinical trials?
Clinical trials for gene therapy for hemophilia, such as those involving Hemgenix, have shown high success rates, with many patients experiencing sustained production of clotting factor IX and a significant reduction in bleeding episodes.
| Key Points | Details |
|---|---|
| Introduction to Gene Therapy | Terence Blue becomes the first patient in New England to receive gene therapy for hemophilia B, called Hemgenix, at Brigham and Women’s Hospital. |
| Living with Hemophilia | Blue has managed hemophilia since childhood, requiring regular injections of clotting factor. New synthetic factors have improved his quality of life over the years. |
| The Gene Therapy Procedure | The therapy uses a modified virus to deliver a corrected gene to liver cells to produce clotting factor IX, potentially reducing the need for regular injections. |
| Therapy Effectiveness | Initial results show Blue’s factor IX levels improved significantly post-treatment, indicating success in the therapy. |
| Market Challenges | High costs and market pressures pose challenges for gene therapy adoption, with some therapies withdrawing due to low patient uptake. |
| Future of Gene Therapy | Despite the challenges, optimism exists for further advancements in gene therapies across various conditions, including hemophilia. |
Summary
Gene therapy for hemophilia is revolutionizing treatment options for patients like Terence Blue, who have spent years managing their condition with regular injections. With the introduction of Hemgenix, patients may experience significant improvements in their health and quality of life, reducing the dependency on constant treatment and offering hope for a more normal life. As research continues and more therapies become available, the potential for lasting solutions to hemophilia is becoming real, providing both clinicians and patients with optimism for the future.
You may also like
Archives
Calendar
| M | T | W | T | F | S | S |
|---|---|---|---|---|---|---|
| 1 | ||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| 23 | 24 | 25 | 26 | 27 | 28 | |